Medical devices or IVDs are often designed for multiple user groups. In this article, we would like to explain the challenges medical devices face in user interface design as a result and how these can be addressed.
Do one (or more) of the following challenges apply to you?
- Are you developing a medical device or IVD that will be approved for multiple user groups?
- You want to know how to design the user interface so that all user groups can use the product safely and easily according to its intended purpose?
- It is important to you to think about the regulatory requirements from the very beginning and you want to know which of them have to be considered in this specific case?
- Unnecessarily complicated user interfaces should be avoided. But how do you do this when the tasks and knowledge of the user groups are different?
- Are you interested in illustrative examples that show you how other medical products have been able to solve these challenges?
Then this is the article for you! Let’s dive deeper into the topic together and come back to the surface with concrete solutions.
A few words about the basics
Medical devices must always be designed to be user-centered, according to the MDR. The FDA requires human factors engineering for all high-risk devices. What exactly does that mean?
The MDR provides the answer in Annex I, Article 5b): You must „ give consideration to the technical knowledge, experience, education, training and use environment, where applicable, and the medical and physical conditions of intended users (design for lay, professional, disabled or other users).”
(Source: MDR, Annex I, Article 5b)
The FDA also follows a very similar course here. The definition of “human factors engineering” states: “The application of knowledge about human behavior, abilities, limitations, and other characteristics of medical device users to the design of medical devices including mechanical and software driven user interfaces, systems, tasks, user documentation, and user training to enhance and demonstrate safe and effective use. Human factors engineering and usability engineering can be considered to be synonymous.”
(Source: FDA, Chapter 3.6)
This is also in line with the IEC 62366-1 definition of “usability engineering”: „ Application of knowledge about human behaviour, abilities, limitations, and other characteristics to the design of MEDICAL DEVICES (including software), systems and TASKS to achieve adequate USABILITY“. (Source: IEC 62366-1:2021-08, 3.17)
So, you see: In usability engineering or human factors engineering, the focus is clearly on the user. A fundamental understanding of the capabilities and limitations is essential for product design.
For our basic challenge “One medical device, multiple users”, this now means: A medical device and its user interface must be designed, tested and validated for each user group that could cause an unacceptable risk during use.
So, to achieve that, you need to find out this information about your users:
- What are the different characteristics of the user groups?
- What previous experience and knowledge levels need to be considered?
- What are the (possibly different) tasks, processes and procedures?
- In the case of products that are also to be approved for laypersons, the following also applies: Laypersons and experts must arrive at the same interpretation of the results – how can this be ensured?
Usability Engineering and Human Factors Engineering: The user groups are always part of the foundation
In order to develop a product for several user groups, you must therefore always know these user groups in detail. Accordingly, the following is not surprising: In Human Factors Engineering according to the Guidance Document “Applying Human Factors and Usability Engineering to Medical Devices”, as well as in IEC 62366-1, the definition of the intended user groups is always an essential part of the foundations on which the further process is built.
In Human Factors Engineering, the first step is called: „Define intended users, use environments and user interface“.
This is also the basis of the usability engineering process according to IEC 62366-1. Here, the step on which all others are based is called “Prepare Use Specification”. In addition to the intended medical indication, the intended use environment, the intended body part or tissue type on which the product is to be used, the functional principle of the product and the intended patient groups, the intended users must also be defined and documented.
This is done in a User Profile. In subsection 3.29, IEC 62366-1 defines the required user profile as follows: „ summary of the mental, physical and demographic traits of an intended USER GROUP, as well as any special characteristics, such as occupational skills, job requirements and working conditions, which can have a bearing on design decisions “ (Source: IEC 62366-1:2021-08; 3.29)
So if you want to develop for multiple user groups, you need multiple user profiles. But these are only part of the basics. You also need a basic understanding of the context of use.
Basic understanding of the context of use
How is the context of use composed? From these factors:
- The users
- Their goals
- Their tasks
- Their resources
- The use environment
To develop for multiple user groups, you need to find out the following for each user group in a context of use analysis:
- What exactly is the task that the user is performing?
- What information and materials does he need for successful execution?
- What are the content-related processes? (What is the user doing? How long does he do it? How often does he need to perform something?)
- What is the timing of the task?
- What are the spatial conditions and how do they influence the execution of the task?
- What are the motivations and needs of the user?
At best, they do this through field observations and interviews. This knowledge gained here is essential for designing the user interfaces for the respective user groups.
Here you will also find out whether your user groups have very similar or very different tasks. We have now defined solution approaches for both cases, in which your user interface design or the concept of your product can go.
Solution 1: Configurable user interfaces and products for user groups with the same tasks
Do your user groups have the same tasks they want to accomplish with your product? Then customizable products or configurable user interfaces are often the best solution.
This means: there is a basic product or user interface that is designed to be operable by all user groups. However, there is an opportunity to have the product customized to meet the requirements or ergonomics of different user groups.
For example, a user interface could have add-on user interfaces for different roles and their tasks in a hospital. For example, a nurse might want to add a different user interface than a chief of staff.
There are several ways to adapt a product to a user’s ergonomics. From electrically adjustable nursing beds to an adjustable cuff on a blood pressure monitor, there are countless solutions here.
Solution 2: Different user interfaces for different user groups
If the user groups have different core tasks, each user group needs a user interface tailored to its own needs and tasks.
Take, for example, an application that allows doctor visits to be mapped completely digitally. This could work through video telephony, patient records and an appointment booking system. Now, doctors and patients have very different tasks within this application. The user interface for the “patients” user group would need to allow them to book and cancel appointments, download prescriptions and other documents as needed, and retrieve diagnoses and important information as necessary. The “doctors” user group would need the following capabilities in a user interface, among others: Issue prescriptions, have a complete view of appointments, view and edit patient records, etc.
The requirements, tasks, workflows and processes are fundamentally different here and each require a user interface adapted to the user group.
Solution 2: Different user interfaces for different user groups
If the user groups have different core tasks, each user group needs a user interface tailored to its own needs and tasks.
Take, for example, an application that allows doctor visits to be mapped completely digitally. This could work through video telephony, patient records and an appointment booking system. Now, doctors and patients have very different tasks within this application. The user interface for the “patients” user group would need to allow them to book and cancel appointments, download prescriptions and other documents as needed, and retrieve diagnoses and important information as necessary. The “doctors” user group would need the following capabilities in a user interface, among others: Issue prescriptions, have a complete view of appointments, view and edit patient records, etc.
The requirements, tasks, workflows and processes are fundamentally different here and each require a user interface adapted to the user group.
Special case: IFUs – A design that all user groups understand
Since different user groups do not receive different IFUs and usage instructions, these must therefore be understandable for all user groups. You must also be able to prove this in the summative evaluation or human factors validation.
The easiest and most accepted way to prove this is through summative user testing. Here, the recommendation is to test the IFUs formatively to be prepared for the final evaluation. A second recommendation is that the final user tests on IFUs can and should be conducted before the rest of the summative evaluation.
Fast and pragmatic user tests for safe medical devices – for every user group
Once you have the basic understanding of users and usage contexts and have decided on one of the solution options above, iteratively test design solutions with real users and then adapt them to the feedback from user testing. In this way, with each iteration, you will get closer to the solution approach that enables the respective user group to use the product without critical user errors and effectively.
Here, you should be open to the fact that multiple user groups may need multiple user interfaces (or prototypes at this stage).
Formative evaluations are a suggestion from both FDA and MDR on how you can make products better in a user-centric way. Especially for a product with different user groups, testing is important because.
- all tasks and needs must be understood in depth
- the critical operating errors must be found out for each user group
- these critical user errors must then be eliminated from the design so that the final testing of your product is positive and the product can be approved.
Already in the formative evaluations it is very important that you have defined your user groups very precisely and recruited them correctly. This is the only way to obtain valid results!
Does each user group need to be included in the summative evaluation or human factors validation?
Yes, for each Hazard-Related-Use-Scenario (i.e., each scenario that can directly or indirectly lead to a hazard), you must demonstrate the operating safety with the respective user group in a final evaluation of your product. However, IEC 62366-1 says that several User Profiles can be combined into one User Group for the purpose of usability testing. (Source: IEC 62366-1:2021-08; 5.7.1)
You must note here that you must justify how the characteristics of the test participants are representative of the intended User Profiles. (Source: IEC 62366-1:2021-08, 5.7.3e).
You want examples?
Now we have answered the initial questions. The following are examples from the world of medical devices to show you how to solve the above challenges in practice.
You don’t need any examples? Then feel free to jump directly to our conclusion. For everyone else: Have fun with our excursions into different product worlds.
Example 1: The hearing contact lens – Customizable ergonomics
There are many medical devices with flexible parts that affect ergonomics. One such product is the hearing contact lens. This is a hearing aid that is adapted to the patient’s ergonomics by the hearing care professional and is intended to be used for hearing loss of various degrees of severity. So here we have an example that fits our solution suggestion 1: A product with customization capabilities.
The hearing contact lens is designed to adhere directly to the eardrum. The vibrating element, microphone, sound processor have to be adjusted to the individual user. But the overall size of the hearing contact lens can also be tailored to fit the user precisely.
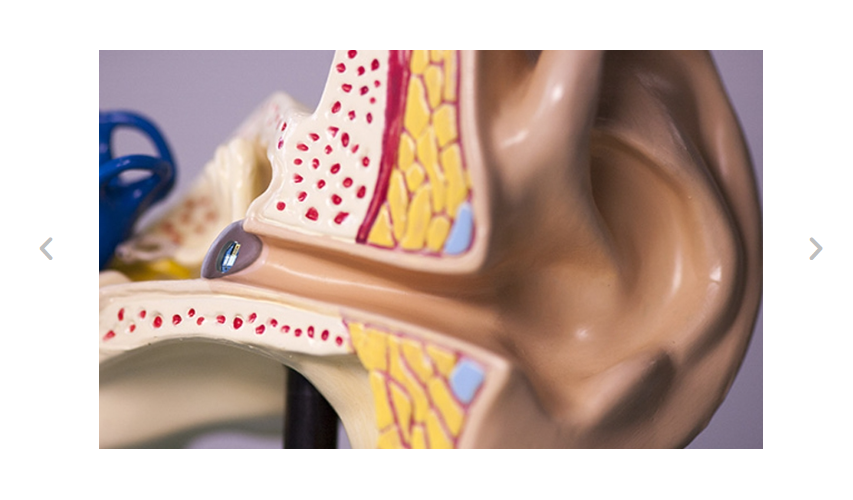
(Source: Screenshot Auric.de)
Example 2: My BIGApp – One App, Multiple User Interfaces
The “meine BIGApp” enables BIG customers to contact their own health insurance company quickly and without red tape. Here we have a case of solution 2 described above: multiple user interfaces for multiple users. There are the accesses for customers and the user interfaces tailored to them, and the accesses for the health insurance company.
The tasks here are of course very different for the mentioned user groups. But even the very large group of “customers” consists of many different users with many different levels of knowledge and technical understanding.
Here, we were able to measurably improve the usability and UX of the customer-user interface with iterative user tests, thus creating an effective and easy-to-use experience for all users, regardless of their prior technical knowledge.
If you are interested in the project, you can find a more detailed report about it in our Case Study for the “MeineBIG App“.
Conclusion
If your medical device has multiple user groups, you need to get to know them deeply in order to tailor the product to their needs and ensure that the product is safe to use. IFUs must be equally understandable to all user groups.
When it comes to user interface design, you have two basic choices:
- If the user groups have the fundamentally same task, then you can design a user interface that is configurable.
- If your user groups have different tasks, then you need to design a separate user interface for each user group that is tailored to their needs.
Do you have questions about designing your user interface for multiple user groups or regulatory requirements?
Feel free to get in touch via our contact form and arrange a free introductory meeting with us.
Of course, you can also write us your questions in the comments. We are very much looking forward to your feedback!



0 Comments