Anyone who wants to have several medical devices validated in summative usability evaluations or human factors validations often faces the following problem: these studies cost a lot of time, money and other resources.
In many cases, the solution is to combine the studies of several medical devices. In this article, you can find out under which conditions and how exactly this works.
We hope you enjoy reading it!
A note before we start
The term “Combined Usability Studies” is not part of the standards and norms. It originates from us and is our proven solution proposal for the pragmatic implementation of mandatory summative evaluation or human factors validations for several medical devices.
What is a Combined Usability Study?
A final and evaluative usability study in which several medical devices are tested together or sequentially to demonstrate that the use of the devices is free of unacceptable risks.
Products whose usability studies can be combined could be the following in this context:
- Two very similar medical devices
- A medical device and other associated products
- A medical device and the associated software
Under which circumstances can I conduct a combined study?
The final validation of multiple medical devices can be combined in certain cases, especially if the devices have similar characteristics or requirements. Here are some key factors that should be considered:
- Similarity of devices: If the medical devices have similar intended uses, it may be possible to combine validation. This is particularly relevant if they have similar risk profiles and quality requirements.
- Similar test requirements: If the devices require the same or very similar tests to confirm their safety and efficacy, combined validation may be more efficient.
- Same user group: If you have several products with the same or very similar users, you can combine the studies here.
What else should you keep in mind?
You should pay particular attention to:
- Risk management: The risk assessment should be carried out for each product individually as well as for the group of products as a whole. This ensures that all specific risks are adequately addressed.
- Documentation and traceability: Thorough documentation is crucial to ensure the validity and traceability of the test results. In a combined validation, it must be clear which data belongs to which product.
- Safety first: Combining validations can be cost-effective, but it must be ensured that this does not compromise the safety or efficacy of the products.
In practice, the decision whether to perform a combined validation should be based on a thorough assessment of the above factors and in compliance with all relevant regulatory requirements. It is also advisable to seek advice from experts or regulatory authorities in cases of uncertainty or complexity.
An example of a combined usability study
This example is based on the following: You have two medical devices that are to be used by the same intended user group. So you decide to save resources by:
- Setting up a simulated usage environment once and preparing a usability lab
- Recruiting users only once
- Training your team responsible for the implementation only once
This is how a combined usability study for two products with the same user group might look, for example:
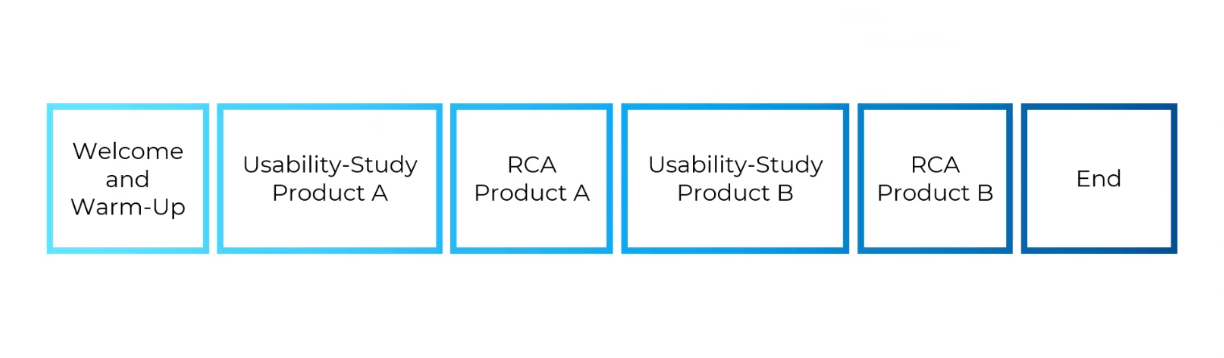
- welcome the participant and obtain all signed data protection documents
- you carry out the usability study for product 1
- the root cause analysis for product 1 follows
- then continue with the usability study for product 2
- then follows the root cause analysis for product 2
- at the end you say goodbye to your participant
Of course, these are just examples. Of course, there are many more possibilities. However, it would go beyond the scope of this article to list them all.
Would you like to find out more?
Then you can find our FAQ on the subject here. We have answered the 12 most important questions on the subject.
Conclusion
Wherever you can meaningfully combine studies, you should do just that. There are many different ways to conduct a combined usability study.
Do you want to know whether a combined study is worthwhile in your case? Let’s find out together! Get in touch using our contact form. We look forward to hearing from you.