Optimized by experts: Usability engineering for your medical devices!
Get your medical device or IVD approved pragmatically and efficiently with us
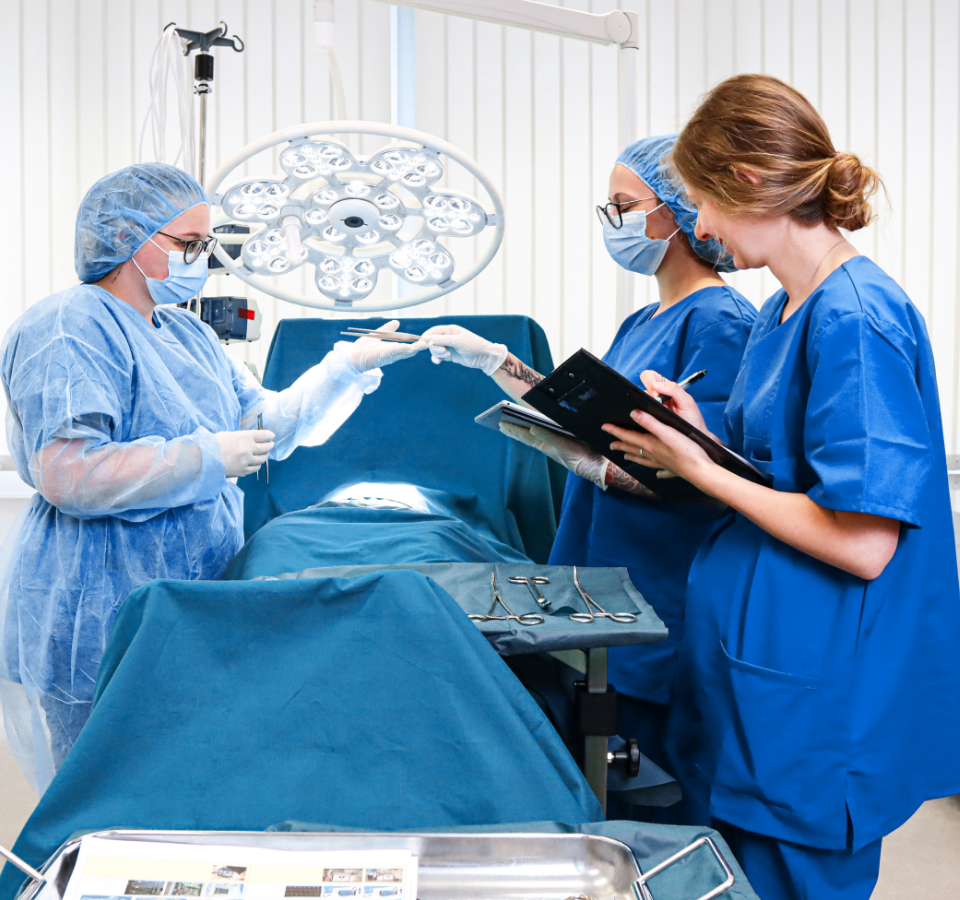
Overview of the benefits
- Reach your goal efficiently with our process: From concept to summative evaluation (including the correct documentation documentation), our step-by-step process guides you through usability engineering.
- Support for over 300 medical devices already: With this process, we have already been able to guide more than 300 medical devices toward a safe and easy to use experience.
- TÜV-certified QM system according to ISO 13485: With our ISO 13485 QM certification for usability engineering, testing and documentation of medical devices, we know your requirements and actively help you meet them.
- Best equipped: Our MedLab, UX labs and equipment allow you to simulate your use case and conduct your usability testing smoothly.
- Recruit the right users: Our user databases cover a wide range of users, from laymen to expert users – if your ideal users are not included, our recruiting team will take care of it for you! This saves you valuable resources.
- Meet the regulatory requirements of multiple markets: Medical devices and IVDs are often approved internationally. We know exactly what is required in each of the markets you target. Our location in the USA also facilitates FDA approval.
















How usability engineering works with us:

0. Gap analysis
We work with you to understand where you are with your product and what gaps we need to fill. Then we make the process as lean as possible to guide you effectively.

1. Use Specification
We identify who your users are, what their tasks are, and lay the groundwork for testing the summative evaluation.

2. Risk analysis
We help you identify critical hazards, control them, and eliminate them as much as possible through design. Always with a strong focus on controlling the risks of use.

3. User requirements
We define what your product must do in order to be used safely by its intended users in accordance with its intended purpose.
4. Design
We define the requirements in a user interface design and rethink your design so that in the end your product is not only compliant with the regulations, but also inspiring.

5. User testing
The user interface design is iteratively tested and evaluated with real users. We document the results so that they are suitable for your usability engineering file.
6. Interations
Iterations follow until your medical device or IVD is free of critical operational errors and truly delights your users.

7. Summative Evaluation
In a summative evaluation, we prove that your product is safety to use. We perform final testing for you according to MDR and FDA requirements in the USA.

8. The correct documentation
In the end, you will have a complete usability engineering file or human factors report, proving that all usability requirements for your product have been met.
What do our customers say about us?

„Custom Medical has been helpful in depth. It’s given us a lot of advices for creating the relevant usability engineering file in addition, to have a nice user interface. To having someone available via videoconference or email can help us with usability engineering evaluation related questions of what the best practices are and how they are applied in the study. And I think that’s Custom Medical apart from anyone else.“
Priscila Panigoro | Regulatory Affairs @Aero Pump
„It was easy to work with Custom Medical. Their structured approach to projects resulted in the successful completion of our project. We would recommend Custom Medical to companies looking for a reliable and structured partner in usability engineering, especially medical device companies facing the issue of summative evaluation.“
Clemens Winkler | Chirurg @Fasciotens


„Working with Custom Medical was very relaxed. […] I would recommend Custom Medical to anyone who does not have enough usability engineering resources in-house. The advantage of Custom Medical is that they not only take care of product validation, but also help improve the UX design of a product.“
Ludwig Wildner | Chief Technology Officer @PreciPoint
Why we are the perfect partner for your usability engineering needs
-
- We are specialized in medical devices and IVDs: QM system according to ISO 13485, TÜV-certified Medical Device Experts and equipment perfectly tailored to medical devices and IVDs – with us you have a partner who knows his stuff!
-
-
- Fast project execution: We respond quickly to your request and keep the implementation as efficient as possible.
-
-
-
-
- The correct documentation: We understand the requirements of all target markets and what the regulatory agencies are looking for. Our documentation will make the approval process much easier for you.
-
-
- User recruitment: We always find exactly the right users for your tests. Our extensive database includes everything from laymen to health care experts.
- Technology and Equipment: Our laboratories allow you to easily reproduce your usage environment – from the doctor’s office to the operating room, everything can be simulated. With the right camera and sound equipment, you can always keep an eye on the studies – live or recorded.
- Our usability engineering results in excellent design: In addition to five Red Dot Design Awards in a row, we have also won the German Design Award. Maybe your product will be our next award winner!

Certified for excellence

Let’s get started!













