Post-market surveillance for medical devices
Post-market surveillance (PMS) for medical devices is crucial for the safety and performance of products after their market launch. It serves to identify potential risks at an early stage, improve the products, and fulfill regulatory requirements.
At Custom Medical, we offer you comprehensive support in developing and implementing efficient PMS measures. This includes regulatory advice, the development of customized PMS processes, implementing PMS plans, and regular execution.
Our expertise in PMS
Multidisciplinary team
Our team of experts in Human Factors and Regulatory Affairs has many years of experience in the medical device industry. This expertise enables a holistic product view and a comprehensive PMS strategy.
Customised solutions
We offer customized PMS solutions tailored precisely to your needs and the special features of the medical devices in question. This gives you specific and relevant results.

Resources for your needs
Whether you need short-term resources or longer-term support, we can provide the resources required for your post-market surveillance both immediately and sustainably.
What we can do for you
Process development
We set up the necessary processes for post-market surveillance for you and ensure that they fit perfectly into your existing processes.
Data collection and evaluation
We collect the data you need for successful post-market surveillance. This frees up your capacity for other tasks.
Regular reporting
We prepare regular reports for you and consult with you based on the results.

Do you have a specific project you would like us to help you with?
You can get to know us in a initial consultation with our experts.

How we take care of the PMS for your medical device
01
Kick-off
At the kick-off meeting, we get an overview of your products and company structure, get to know all stakeholders, and define project goals together. We draw up a schedule, clarify project roles, and exchange all relevant information. In the end, both sides are well prepared for the project.


02
Workshop
In a joint workshop, we analyze your current challenges and existing structures as a starting point for the post-market surveillance process. We work out your specific needs to fulfill the requirements and develop an efficient implementation strategy. At the end of the workshop, we will have a clear overview of your available means and resources for the PMS and a basis for creating the process description.
03
Creation of the process description
In accordance with the specifications of your quality management system, we create the process description for post-market surveillance and templates for documentation and recording that meet the requirements of the MDR and IVDR. The processes are developed in close consultation with you. In a final meeting, we present the process and the templates to all those involved in the project and provide application tips and advice.


04
Development of product-specific PMS plans
We work with you to create product-specific PMS plans. In doing so, we jointly determine which roles and tasks we will take on in the project and create a schedule that meets your needs and your PMS plan.
05
Performing the PMS
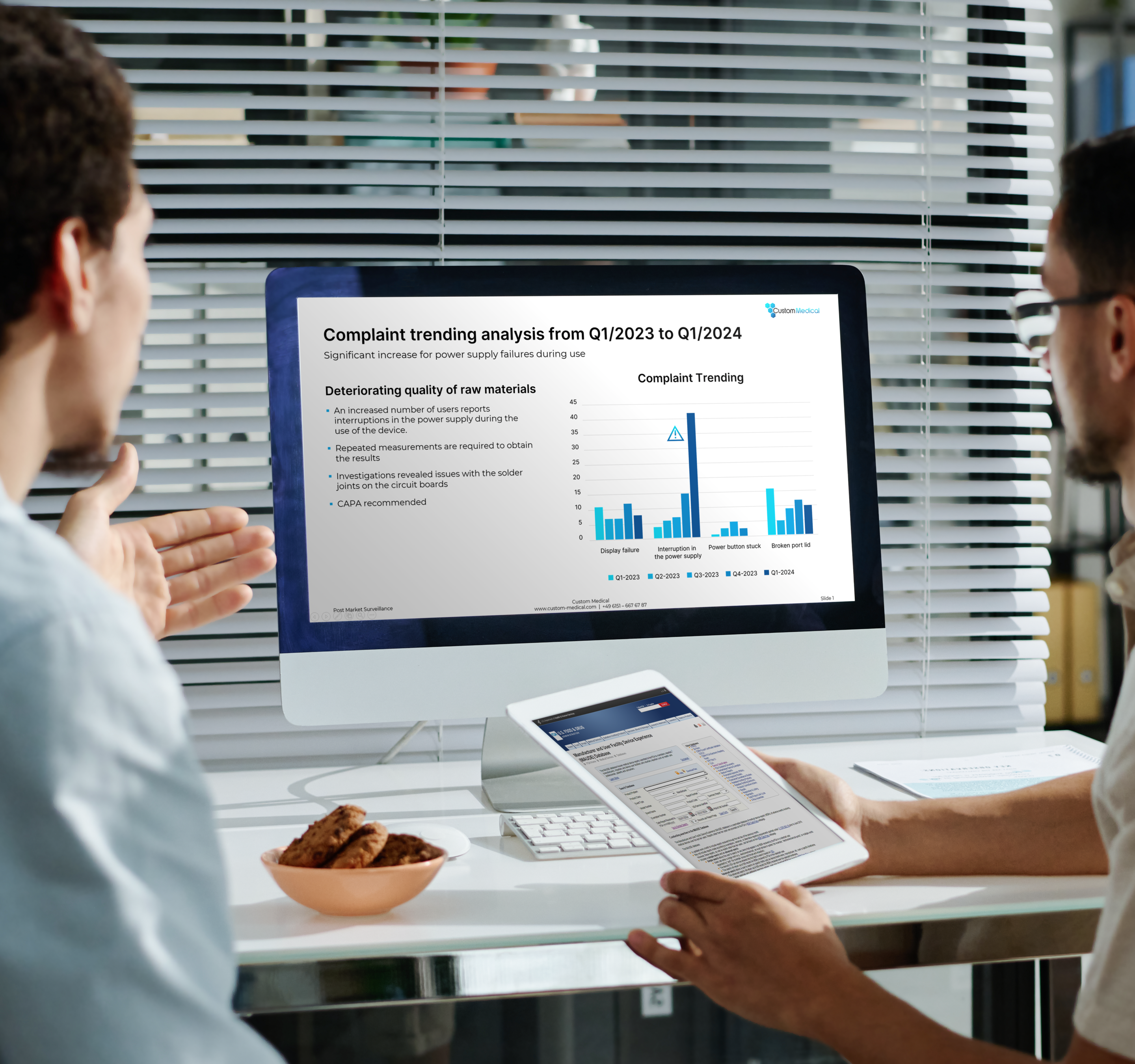
We collect and systematically categorize the data in accordance with your products’ PMS plans. We create a detailed list of the data, such as a summary and initial assessment. We identify areas for improvement so that you can react in good time to ensure the continued safety of your product.

06
Präsentation der Ergebnisse
We present the collected data and the preliminary assessment to your risk management team. We discuss the final assessment together and adjust the evaluation if necessary. If the results give rise to new requirements for your product’s PMS plan or improvement measures are required, we will develop these together and integrate them into the PMS plan accordingly.
07
Regular reports and updates
With our PMS subscription, your PMS data is updated regularly. We take care of timely data collection and data analysis and coordinate the consultation dates and adjustments according to the results. This frees up your capacity for other tasks!

Frequently asked questions about post-market surveillance for medical devices
What are the legal requirements for PMS of medical devices?
The legal requirements for PMS of medical devices vary depending on the region, but in the European Union (EU), they are governed in particular by Regulation (EU) 2017/745 on medical devices (MDR) and Regulation (EU) 2017/746 on in vitro diagnostic medical devices (IVDR). These regulations require manufacturers to set up and operate a PMS system, prepare regular PMS reports (PMSR) and periodic safety reports (PSUR), and report safety incidents to the competent authorities. The Food and Drug Administration (FDA) regulates PMS in the USA through various laws and guidelines, including 21 CFR Part 822 (Quality System Regulation).
What data sources are used for PMS and how is this data collected?
These are some of the common sources:
-
- Clinical trials: Data from post-marketing clinical trials.
- Incident reports: Reports of adverse events from healthcare professionals, patients, or users.
- Registers and databases: National and international registers and databases that collect information on medical devices and their use.
- Literature research: Scientific publications and specialized literature.
- Market feedback: Feedback from users, distributors, and patients, including complaints and returns.
- Many other sources such as internal data from production and quality control, from service technicians/service reports, social media research, trade fairs, and conferences.
How often should PMS reports be prepared and submitted?
The frequency of preparation and submission of PMS reports depends on the type and risk profile of the medical device. In the EU, manufacturers of high-risk devices (Class III and Class IIb implantable devices) must prepare and submit annual Periodic Safety Reports to the relevant Notified Body. The frequency may vary for other devices, with the report typically being updated every two to five years. In the US and other regions, requirements may vary, and it is essential to consider the specific regulatory requirements of each market.
What are the most important components of a PMS system?
- PMS-Plan: A documented plan that describes the strategy and methods for monitoring the product.
- Data collection: Methods for systematically collecting safety and performance-related data.
- Data analysis: Methods for analyzing the collected data to identify trends and potential problems.
- Risk management: Integration of PMS data into the risk management system to continuously assess and mitigate risks.
- Reporting: Prepare reports for the competent authorities and notified bodies.
- Communication: Informing users, patients, and other stakeholders about relevant safety information and product changes.
We want to get to know you and your product!
















